
Credit: Adrianna Trusiak
by Adrianna Trusiak
Location: Toolik Field Station, Alaskan Arctic
Red and orange across the Arctic
In the environment iron is easy to identify due to its color. Specifically, on the surface iron is exposed to oxygen in the atmosphere and oxidized, forming red-orange precipitates visible to the naked eye. Across the arctic landscape, these red-orange precipitates can be found near rivers and streams, in soils, and even on the snow banks. When iron is below the surface, in an oxygen poor environment, iron is reduced and it is actually invisible. The interplay between iron in its invisible, reduced form and red-orange, oxidized form plays a role in the production of carbon dioxide (CO2) in arctic soils and soil waters.

Figure 1. Red-orange oxidized iron precipitates across the arctic landscapes.
Credit: Adrianna Trusiak
In the general chemistry when learning about redox, we are taught that the reduced iron oxidation produces oxidized iron. However, in addition to oxidized iron, highly reactive oxidants are produced. Those highly reactive oxidant are capable of oxidizing organic carbon to CO2. Across the Arctic there are many waterlogged, standing water areas, creating low oxygen condition in the soils. Low oxygen conditions in the soils lead to the accumulation of reduced iron. However, once those soils (and reduced iron in them) are exposed to oxygen due to disturbance, for example rain or someone (or some animal) stepping on the soils; that reduced iron is oxidized to oxidized iron. In my Ph.D. research I found that during iron oxidation in arctic soil, reactive oxidants oxidize organic carbon to produce CO2.[1] This previously unrecognized pathway of CO2 production from iron oxidation in arctic soils and soil water, can produce as much CO2 produced through microbial respiration in arctic surface waters.[2]

Researchers at Toolik Lake
Credit: Adrianna Trusiak
To study the iron oxidation and consequent production of CO2, together with colleagues from the University of Michigan, I spent three summers in the Arctic collecting and analyzing soil waters. In the mornings out team would go out to the field to collect soil waters, either walking, driving a truck, or on special days flying in a helicopter to more remote locations far from Toolik Field Station (where the Arctic LTER in based out of). The sample collection for this study involved sampling water without introducing oxygen from the atmosphere into the water sample- let me just say it involved a lot of patience and slow manipulations of sample syringes. In addition to collecting water for analysis and experiments back in lab at the field station, we measured pH, conductivity, and temperature of the soil water to better understand soil water chemistry. Sometimes in the field we also quickly checked for presence of reduced iron in soil water by mixing some of the soil water with ferrozine- a reagent that turns purple if reduced iron is present!
Back in the lab, the experiments would start! Majority of the work needed to be done in a glove bag where there is no oxygen in the atmosphere, and thus no oxygen is introduced to the soil waters. First thing after getting back from the field, we filtered the soil waters to remove any microbes that could produce CO2 through respiration. Filtered soil waters were split for measurements (iron, reactive oxidants, and CO2) and treatments (no oxygen added and oxygen added). We simulated oxidation of the soil water by introducing controlled amount of oxygen to the water and measuring changes in iron, and reactive oxidant, and CO2 production from that oxidation. After a long day of sampling in the field and measurements back in the lab, we would still get some sunlight after a long day of work thanks to 24 hours of sunlight during summer in the Arctic!

Figure 3. Soil waters had to be filtered in a oxygen-free glove bag back in the lab at Toolik Field Station to avoid oxidation of reduced iron before the start of experiments.
Credit: Adrianna Trusiak
The Arctic is red hot
Human activity is increasing the amount of CO2, a heat-trapping gas in the atmosphere, leading to the warming of our planet. The Arctic is warming twice as fast as the rest of the planet and, as a result, tremendous amounts of organic carbon that have been frozen for thousands of years are thawing[3]. Thawed organic carbon can be converted to CO2 through biological processes like microbial respiration, and through chemical processes including sunlight oxidation of organic carbon[4]. The conversion of the dissolved form of organic carbon to CO2 in soil and surface waters is up to 40% of the net global carbon land-atmosphere exchange in the Arctic[5]. Thus, CO2 production from the newly thawed organic carbon could have a large impact on the carbon cycle and accelerate climate change[6]. Understanding the processes controlling CO2 production in arctic soils is crucial for predicting the influence of arctic warming on the future climate.
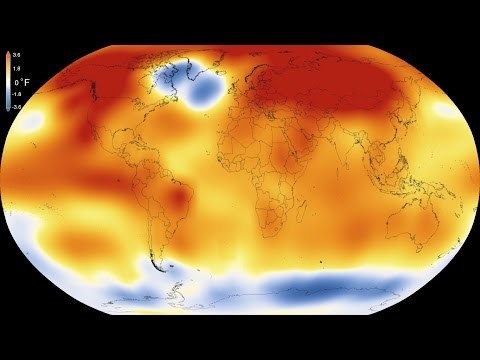
Figure 4. The Arctic is warming twice as fast as the rest of the planet.
Credit: NASA
References
[1] Trusiak et al. 2018, GCA
[2] Page et al. 2013, ES&T
[3] Osborner et al. 2018. Arctic Report Card.
[4] Cory et al. 2014, Science.
[5] McGuire et al. 2009. Ecological Monographs. 79: 523–555.
[6] MacDougall et al. 2012. Nature Geoscience. 5:719-721.

Author Biography: Adrianna Trusiak is a doctorate candidate in Professor Rose Cory’s lab in the Department of Earth and Environmental Science at the University of Michigan. Adrianna spent four field seasons in the Alaskan Arctic collecting soils and soil waters and running experiments and sample analysis at Toolik Field Station. Outside of the lab, Adrianna enjoys volunteering with animals, exploring nature areas, and watching movies.










